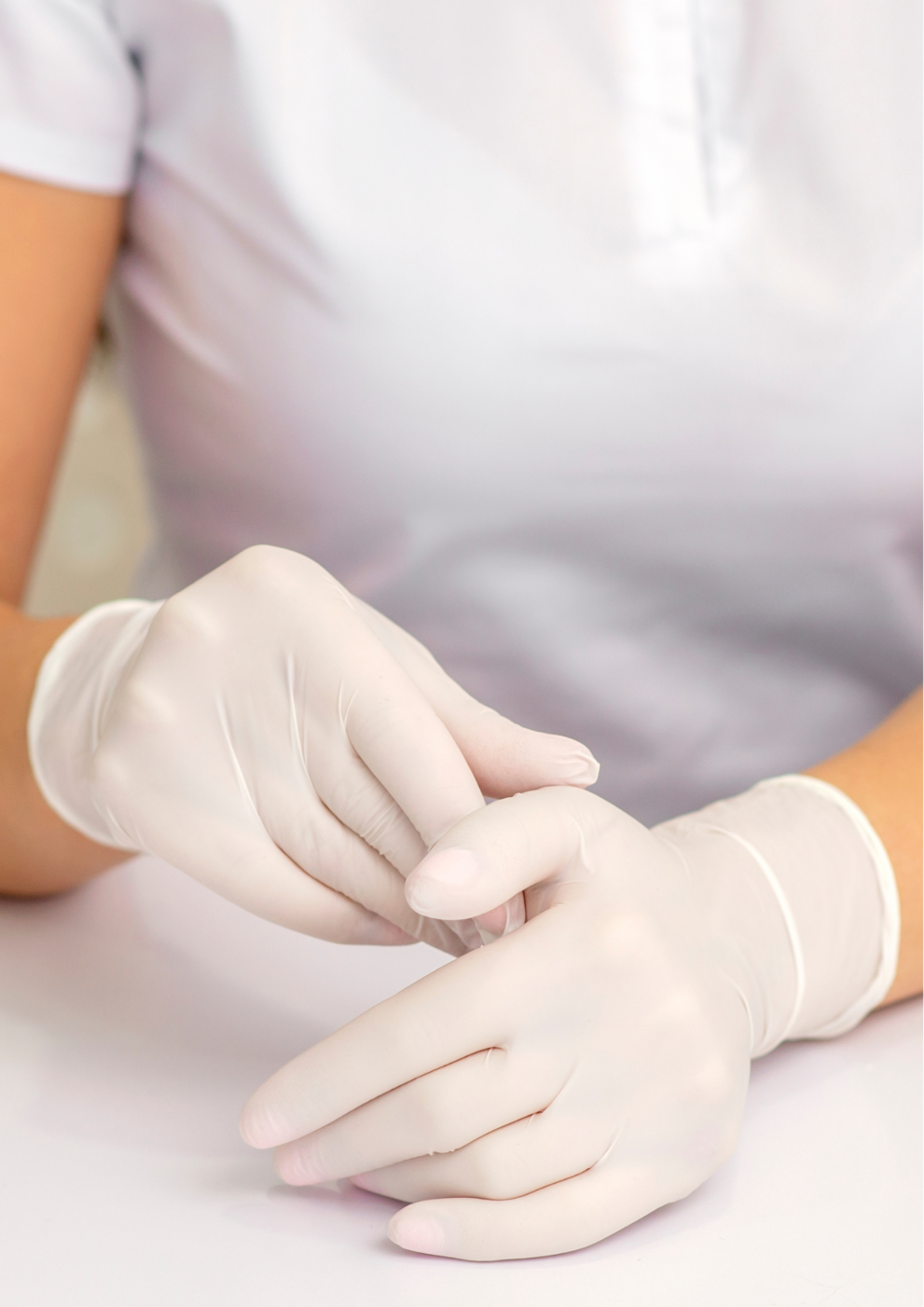
Product standards and certifications
REGULATORY REFERENCES
Medical Devices (MD)
Products bearing the Medical Device CE marking must meet all the essential and applicable requirements laid down in Annex I of EU Regulation 2017/745 (former Directive 93/42/EEC) on medical devices. The Directive defines a “medical device” as any instrument, apparatus, implant, software, substance or other product, used alone or in combination, including accessories like software intended by the manufacturer to be used specifically for diagnostic and/or therapeutic purposes and necessary for the correct functioning of the device itself, intended by the manufacturer to be used on humans for:
- diagnosis, prevention, control, treatment or alleviation of disease;
- diagnosis, monitoring, treatment, alleviation or compensation of an injury or disability;
- investigation, replacement or modification of the anatomy or of a physiological or pathological process or state;
- control of conception which does not achieve its principal intended action by pharmacological, immunological or metabolic means, in or on the human body, but which may be assisted in its function by such means.
MDs are divided into four classes, according to the characteristics and the degree of risks associated with them, as laid down in Annex IX of Directive 93/42. The classes, starting from the lowest risk class, are divided into:
- Class I MD – low-risk devices. Devices that fall into this class do not require the intervention of a notified body (apart from sterile devices and/or devices with a measuring function), and must be registered with the competent local authorities.
- Class IIa and llb MD – medium-risk devices for which the intervention of a notified body is required.
- Class III MD – high-risk devices for which the intervention of a Notified Body is required.
REGULATORY REFERENCES
MD (Medical Devices)
(Reg. EU 2017/745)
UNI EN 455-1
Specifies requirements and provides the test method for single-use medical gloves in order to determine freedom from holes.
UNI EN 455-2
Specifies requirements and provides the test methods for physical properties of single-use medical gloves, in order to ensure that they provide and maintain in use an adequate level of protection from cross contamination for both patient and user.
UNI EN 455-3
Specifies requirements for the evaluation of biological safety for medical gloves for single use. It gives requirements for labelling and packaging of gloves, as well as the disclosure of information relevant to the test methods used.
UNI EN 455-4
Specifies the requirements for the shelf life of single-use medical gloves.
REGULATORY REFERENCES
Personal protective
equipment (PPE)
Products bearing the Personal Protective Equipment CE marking must meet all the applicable essential requirements set forth in Annex I of EU Regulation 2016/425 on personal protective equipment, which repeals Council Directive 89/686/EEC. Annex I to the aforementioned Regulation defines the risk categories by which PPE is intended to protect users, and in particular:
CATEGORY I
Category I includes only the following minimal risks:
a. superficial mechanical injuries;
b. contact with mildly aggressive cleaning products, or prolonged contact with water;
c. contact with hot surfaces that do not exceed 50°C;
d. eye injury due to exposure to sunlight (other than injuries due to staring at the sun);
e. non-extreme atmospheric conditions.
CATEGORY II
Category II is defined by exclusion, and refers to risks other than those listed in Categories I and III.
CATEGORY III
Category III only includes risks that can cause very serious consequences, such as death or irreversible damage to health, with regard to the following:
a. dangerous substances and mixtures;
b. oxygen-deficient environments;
c. harmful biological agents;
d. ionizing radiations;
e. high-temperature environments having effects comparable to those of an air temperature of at least 100°C;
f. low-temperature environments having effects comparable to those of an air temperature of -50°C or lower;
g. falls from above;
h. electric shock and work with live wires;
i. drowning;
j. high pressure jets;
k. getti ad alta pressione;
l. bullet or knife wounds;
m. harmful noise.
For risk Categories II and III, the intervention of a Notified Body is required to issue the certification of suitability for the declared degree of protection.
REGULATORY REFERENCES
Personal protective equipment (PPE)
(Reg. UE 2016/425)
UNI EN 374-1:2017
Specifies the terminology and performance requirements of the gloves intended to protect the user against hazardous chemicals. It must be applied in conjunction with UNI EN 420. The standard does not specify the requirements for protection against mechanical hazards.
UNI EN 374-2:2015
Specifies a test method concerning the penetration resistance of protective gloves against chemicals and/or dangerous microorganisms (Acceptable Quality Limit, AQL).
UNI EN 374-4:2014
Specifies the test method for determining the resistance of protective glove materials to degradation by continuous contact with hazardous chemicals.
UNI EN 374-5:2017
Specifies the requirements and test methods of gloves intended to protect the user against microorganisms.
UNI EN 16523-1:2015
Specifies a test method for determining the resistance of protective clothing, gloves and footwear materials to the permeation of potentially hazardous liquid chemicals under conditions of continuous contact. The method is not suitable for the evaluation of chemical mixtures, except for aqueous solutions.
UNI EN 16523-2:2015
Specifies a test method for determining the resistance of protective clothing, gloves and footwear materials to the permeation of potentially hazardous gaseous chemicals under conditions of continuous contact. The method is not suitable for the evaluation of gaseous chemical mixtures.
UNI EN 420:2010
Defines the general requirements and the corresponding test procedures for the design and manufacture of gloves, the resistance of glove materials to water penetration, safety, comfort and efficiency, marking and information provided by the manufacturer applicable to all protective gloves.
UNI EN 388:2016
Specifies the requirements, test methods, brand and information provided by the manufacturer for protective gloves against mechanical risks of abrasion, blade cutting, laceration, perforation and, if applicable, impact. The standard applies together with UNI EN 420.1 developed test methods can also be applied to arm guards.
REGULATORY REFERENCES
Pictographs
To protect consumers and users of personal protective equipment, the European Community requires checks, tests and analyses to ensure that the product is safe and in compliance with current safety regulations.

Compliant with EEC regulations

Certified for protection against microbiological risks, in reference to the UNI EN 374-1 standard

Certified for protection against microbiological risks, in reference to the UNI EN 374-5 standard

Manufactured in EN ISO 9001-certified production facilities

Manufactured in EN ISO 13485-certified production facilities

AQL - Acceptable Quality Level. Less than 1.5 for micro holes with inspection level

Latex-free

To protect from rain and humidity

To protect from heat and direct sun sourceS

Disposable
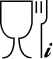
Suitable for contact with food

Recyclable
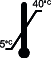
Temperature of use

Medical device